Peroxynitrite Scavenging and Singlet Oxygen Scavenging Antioxidant, Antifungal Activity and Investigation of Natural Metabolites of Pineapple (Ananas comosus) Peels Ethanolic Extract Using GC-MS Technique
Keywords:
Peroxynitrite, Singlet Oxygen Scavenging, Antifungal, Metabolites of Pineapple, Peels, GC-MS TechniqueAbstract
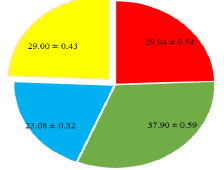
The pineapple has been extensively cultivated due to the high value of the product in the market and high production volume. Harvest has a crucial part played by the ripening stage that affects sensory quality and shelf life, particularly the non-climacteric fruit since they do not ripen after picking. The aim of this investigation was to study antioxidant and antifungal activity of pineapple metabolites based on metabolite profiling method. The results of the small molecule study revealed thirteen compounds which include alpha-Farnesene, (3Z,6Z), Palmitic acid-1,2-13C2, Tricin, Feruloylputrescine, n-Decanal, 2,4-dichlorobenzoic acid, Caryophyllene, 1-o-p-coumaroylglycerol, Chrysoeriol-7-O-glucoside, alpha-Myrcene, N,-p-Coumaroyl-N,fer Antioxidants chemical substances have the capability to prevent degenerative diseases by supplying electrons or electrons to free radicals. Fruit peroxide scavenging Fruit chemical such as crude methanolic extract of the uproot fructus; ethyl acetate, ethanol, and gallic acid (standard) expressed were 709.00±25.45, 645.80±20.00, 673.31±22.10 and 812.00±29.07 while recorded 49.00±3.71, 28.50±2.46, 36.09±2.00 and 43.26±2.19 respectively for Singlet oxygen scavenging. When compared to amphotericin B (Am B) and nystatin (NY), the antifungal activity of methanol and ethanol, which are natural metabolites of pineapple (Ananas comosus) peels, were 25.91±0.35, 21.05±0.29, 35.00±0.58 and 31.64±0.53 in Candida albicans while recorded 15.40 ±0.24, 17.09±0.25, 24.09±0.33 and 22.00±0.31 in Cladosporium herbarum. In the same time recorded 16.70±0.25, 11.98±0.21, 17.00±0.25 and 22.04±0.29 for Trichophyton rubrum. Antifungal activity of Fusarium oxyporum 15.12±0.24, 20.09±0.27, 21.09±0.28 and 27.00±0.41 while record 29.00±0.43, 23.08±0.32, 37.90±0.59 and 29.04±0.54 in Cladosporium herbarum.
Downloads
References
Shui, G., and Leong, L., P. (2006). Residue from star fruit as valuable source for functional food ingredients and antioxidant nutraceuticals. Food Chemistry 97(2), 277–284, 2006.
Cassellis, M., A., R., Pardo, M., E., S., López, M., R., and Escobedo, R., M. (2014). Structural, physicochemical and functional properties of industrial residues of pineapple (Ananas comosus). Cellulose Chem. Technol., 48(7-8),633-641.
Aguedo, M., Kohnen, S., Rabetafika, N. (2012). Composition of by-products from cooked fruit processing and potential use in food products. Journal of Food Composition and Analysis 27(1), 61–69
Ashoush, I., S., and Gadallah, M., G., E. (2011). Utilization of mango peels and seed kernels powders as sources of phytochemicals in biscuit, World Journal of Dairy and Food Sciences, 6 (1), 35-42.
Feumba, D., R., Ashwini, R., P., and Ragu S., M. (2016). Chemical composition of some selected fruit peels. European Journal of Food Science and Technology, 4(4),12-21
Bozkurt, T., Gülnaz, O., and Kaçar, Y., A. (2017). Chemical composition of the essential oils from some citrus species and evaluation of the antimicrobial activity IOSR Journal of Environmental Science, Toxicology and Food Technology (IOSR-JESTFT) 11(10),1-8
Feumba, D., R., Ashwini, R., P., and Ragu S., M. (2016). Chemical composition of some selected fruit peels. European Journal of Food Science and Technology, 4(4), 12-21
Pedreschi, R.; Munoz, P.; Robledo, P.; Becerra, C.; Defilippi, B.G.; van Eekelen, H.D.L.M.; Mumm, R.; Westra, E.H.; de Vos, R.C.H. Metabolomics Analysis of Postharvest Ripening Heterogeneity of ‘Hass’ Avocadoes. Postharvest Biol. Technol. 2014, 92, 172–179.
Monti, L.L.; Bustamante, C.A.; Osorio, S.; Gabilondo, J.; Borsani, J.; Lauxmann, M.A.; Maulion, E.; Valentini, G.; Budde, C.O.; Fernie, A.R.; et al. Metabolic Profiling of a Range of Peach Fruit Varieties Reveals High Metabolic Diversity and Commonalities and Differences during Ripening. Food Chem. 2016, 190, 879–888.
Allwood, J.W.; Cheung, W.; Xu, Y.; Mumm, R.; De Vos, R.C.H.; Deborde, C.; Biais, B.; Maucourt, M.; Berger, Y.; Schaffer, A.A.; et al. Metabolomics in Melon: A New Opportunity for Aroma Analysis. Phytochemistry 2014, 99, 61–72.
Bailly F, Zoete V, Vamecq J, Catteu JP, Bernier JL: Antioxidant actions of ovothiol-derived 4-mercaptoimidazoles: glutathione peroxidase activity and protection against peroxynitrite-induced damage. FEBS Lett. 2000, 486: 19-22.
Beckman JS, Chen H, Ischiropulos H, Crow JP: Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994, 233: 229-240.
Chakraborty N, Tripathy BC: Involvement of singlet oxygen in 5-aminolevulinic acid-induced photodynamic damage of cucumber (Cucumbis sativus L.) chloroplasts. Plant Physiol. 1992, 98: 7-11.
Parijadi, A.A.R.; Putri, S.P.; Ridwani, S.; Dwivany, F.M.; Fukusaki, E. Metabolic Profiling of Garcinia Mangostana (Mangosteen) Based on Ripening Stages. J. Biosci. Bioeng. 2018, 125, 238–244.
Karagiannis, E.; Michailidis, M.; Karamanoli, K.; Lazaridou, A.; Minas, I.S.; Molassiotis, A. Postharvest Responses of Sweet Cherry Fruit and Stem Tissues Revealed by Metabolomic Profiling. Plant Physiol. Biochem. 2018, 127, 478–484.
Jarret, D.A.; Morris, J.; Cullen, D.W.; Gordon, S.L.; Verrall, S.R.; Milne, L.; Hedley, P.E.; Allwood, J.W.; Brennan, R.M.; Hancock, R.D. A Transcript and Metabolite Atlas of Blackcurrant Fruit Development Highlights Hormonal Regulation and Reveals the Role of Key Transcription Factors. Front. Plant Sci. 2018, 9, 1–22.
Montecchiarini, M.L.; Margarit, E.; Morales, L.; Rivadeneira, M.F.; Bello, F.; Gollán, A.; Vázquez, D.; Podestá, F.E.; Tripodi, K.E.J. Proteomic and Metabolomic Approaches Unveil Relevant Biochemical Changes in Carbohydrate and Cell Wall Metabolisms of Two Blueberry (Vaccinium Corymbosum) Varieties with Different Quality Attributes. Plant Physiol. Biochem. 2019, 230–244.
Steingass, C.B.; Dell, C.; Lieb, V.; Mayer-Ullmann, B.; Czerny, M.; Carle, R. Assignment of Distinctive Volatiles, Descriptive Sensory Analysis and Consumer Preference of Differently Ripened and Post-Harvest Handled Pineapple (Ananas Comosus [L.] Merr.) Fruits. Eur. Food Res. Technol. 2016, 242, 33–43.
Ogawa, E.M.; Costa, H.B.; Ventura, J.A.; Caetano, L.C.S.; Pinto, F.E.; Oliveira, B.G.; Barroso, M.E.S.; Scherer, R.; Endringer, D.C.; Romão, W. Chemical Profile of Pineapple Cv. Vitória in Different Maturation Stages Using Electrospray Ionization Mass Spectrometry. J. Sci. Food Agric. 2018, 98, 1105–1116.
Steingass, C.B.; Grauwet, T.; Carle, R. Influence of Harvest Maturity and Fruit Logistics on Pineapple (Ananas Comosus [L.] Merr.) Volatiles Assessed by Headspace Solid Phase Microextraction and Gas Chromatography-Mass Spectrometry (HS-SPME-GC/MS). Food Chem. 2014, 150, 382–391.
Steingass, C.B.; Glock, M.P.; Schweiggert, R.M.; Carle, R. Studies into the Phenolic Patterns of Different Tissues of Pineapple (Ananas Comosus [L.] Merr.) Infructescence by HPLC-DAD-ESI-MS (n) and GC-MS Analysis. Anal. Bioanal. Chem. 2015, 407, 6463–6479.
Sagar, N., A., Pareek, S., Sharma, S., Yahia, E., M., Lobo, M., G. (2018). Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Comprehensive Reviews in Food Science and Food Safety 17(3), 512 -531
Shalini, R. and Gupta, D. K. (2010), Utilization of pomace from apple processing industries: a review. Journal of Food Science and Technology, 47 4), 365-371.
Silva, D., I., S., G. D. R. Nogueira and A.G. Duzzioni, Ind. Crop. Prod., 50, 557 (2013). In: Cassellis, M., A., R., Pardo, M., E., S., López, M., R., and Escobedo, R., M. (2014). Structural, physicochemical and functional properties of industrial residues of pineapple (Ananas comosus). Cellulose Chem. Technol., 48(7-8), 633-641
Werth, M.T.; Halouska, S.; Shortridge, M.D.; Zhang, B.; Powers, R. Analysis of Metabolomic PCA Data Using Tree Diagrams. Anal. Biochem. 2010, 399, 58–63.
Teoh, S.T.; Putri, S.; Mukai, Y.; Bamba, T.; Fukusaki, E. A Metabolomics-Based Strategy for Identification of Gene Targets for Phenotype Improvement and Its Application to 1-Butanol Tolerance in Saccharomyces Cerevisiae. Biotechnol. Biofuels 2015, 8, 1–14.
Downloads
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Current Clinical and Medical Education













